Natural killer cells (also known as NK cells, K cells, and killer cells) are a type of lymphocyte (a white blood cell) and a component of innate immune system.
NK cells play a major role in the host-rejection of both tumours and virally infected cells. Natural killer cells are cytotoxic; small granules in their cytoplasm contain special proteins such as perforin and proteases known as Granzymes.
Upon release in close proximity to a cell slated for killing, perforin forms pores in the cell membrane of the target cell through which the granzymes and associated molecules can enter, inducing apoptosis. The distinction between apoptosis and cell lysis is important in immunology – lysing a virus-infected cell would only release the virions, whereas apoptosis leads to destruction of the virus inside. NK cells are activated in response to interferons or macrophage-derived cytokines.
They serve to contain viral infections while the adaptive immune response is generating antigen-specific cytotoxic T cells that can clear the infection. Patients deficient in NK cells prove to be highly susceptible to early phases of herpes virus infection.
FUNCTIONS OF NATURAL KILLER CELLS
Natural killer (NK) cells are effector lymphocytes of the innate immune system that control several types of tumors and microbial infections by limiting their spread and subsequent tissue damage.
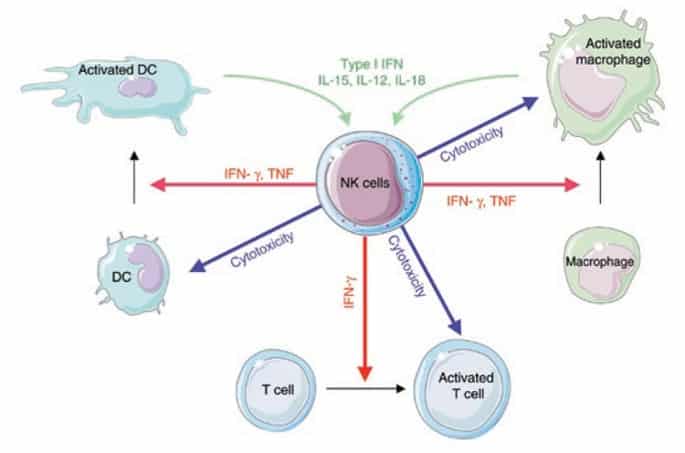
Recent research highlights the fact that NK cells are also regulatory cells engaged in reciprocal interactions with dendritic cells, macrophages, T cells and endothelial cells. NK cells can thus limit or exacerbate immune responses. Although Natural killer cells might appear to be redundant in several conditions of immune challenge in humans, NK cell manipulation seems to hold promise in efforts to improve hematopoietic and solid organ transplantation, promote antitumor immunotherapy and control inflammatory and autoimmune disorders.
HOW NATURAL KILLER CELLS KILL CANCER CELLS
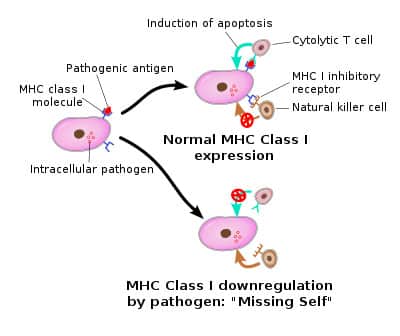
Natural killer (NK) cells are lymphocytes that were first identified for their ability to kill tumor cells without deliberate immunization or activation. Subsequently, they were also found to be able to kill cells that are infected with certain viruses and to attack preferentially cells that lack expression of major histocompatibility complex (MHC) class I antigens.
The recent discovery of novel NK receptors and their ligands has uncovered the molecular mechanisms that regulate NK activation and function. Several activating NK cell receptors and costimulatory molecules have been identified that permit these cells to recognize tumors and virus-infected cells.
These are modulated by inhibitory receptors that sense the levels of MHC class I on prospective target cells to prevent unwanted destruction of healthy tissues. In vitro and in vivo, their cytotoxic ability can be enhanced by cytokines, such as interleukin (IL)-2, IL-12, IL-15 and interferon alpha/beta (IFN-alpha/beta). In animal studies, they have been shown to play a critical role in the control of tumor growth and metastasis and to provide innate immunity against infection with certain viruses.
Also Read: Things You Should Know About Cancer Insurance Policies
Following activation, Natural killer cells release cytokines and chemokines that induce inflammatory responses; modulate monocyte, dendritic cells, and granulocyte growth and differentiation; and influence subsequent adaptive immune responses. The underlining mechanism of discriminating tumor cells and normal cells by NK cells has provided new insights into tumor immunosurveillance and has suggested new strategies for the treatment of human cancer.
NATURAL KILLER CELLS AND PREGNANCY
The endocrine system and the immune system interact closely during implantation and maintenance of pregnancy. One of the most striking examples of this communication is at the level of the decidua (endometrium of pregnancy).
Here, under the influence of sex steroids, there is a dramatic increase of a unique population of lymphocytes, the uterine natural killer (uNK) cells, in early pregnancy. These cells derive predominantly from a subset of peripheral blood NK cells, which under hormonal influence gets recruited to the uterus. In mice, uNK cells play an important role in the development of placental vasculature.
The role of these cells in human pregnancy is still not definitively established; however, they are believed to promote placental and trophoblast growth and provide immunomodulation at the maternal-fetal interface. In contrast to their presumptive role in the maintenance of a healthy pregnancy, uNK cells and peripheral NK cells are dysregulated in unexplained recurrent pregnancy loss.
Herein, we review natural killer cells populations, their changes in number and function in altered endocrine environments during the menstrual cycle and pregnancy, the current data on their potential role in unexplained recurrent pregnancy loss, and mechanisms for potential therapies targeted to NK cell function for this enigmatic disorder.
HOW TO INCREASE NATURAL KILLER CELLS
The enhancement of NK cell function can be accomplished in a variety of ways. For the purposes of this review, we break down the strategies into three major categories:
- Targeting inhibitory signaling pathways and negative regulators of NK cell activating signaling pathways,
- Manipulation of inhibitory/activating receptors expressed by NK cells,
- Cytokine-mediated activation and expansion of natural killer cells. This review will highlight the scientific progress in these 3 areas and discuss how these different strategies are currently impacting NK cell-mediated immunotherapy.
CONCLUSION
Since their discovery more than 3 decades ago, our knowledge about the function of human NK cells has grown exponentially. Once considered a forerunner of the seemingly more sophisticated antigen-specific, memory-bearing adaptive immune system,11 it is now clear that NK cells are highly sophisticated players of the innate immune system with certain recognition features that arrived on the evolutionary scene with primates approximately 400 million years after the birth of adaptive immunity.
Their maturational pathway is strikingly similar to that of T cells, yet the discovery of developmental NK cell intermediates in SLT suggests this is a unique site for NK ontogeny in humans. The phenotypic heterogeneity of NK cells is accompanied by relatively distinct functional attributes, and it now appears that NK cells bridge innate- and antigen-specific immunity via their secretion of cytokines in SLT, while complimenting cytolytic effector T-cell immune surveillance by recognizing pathogens with absent or diminished MHC class I expression.
Whether it is cytokine secretion or cytotoxicity, NK- cell function appears to result from the absolute sum of simultaneous activation and inhibition signals. Although we have gained only a partial understanding of how natural killer cells recognize a target cell as friend or foe, the principles have already enhanced our understanding of how pathogens evade the immune system and how the immune system can be engineered to cure some forms of cancer.